1. INTRODUCTION
Nanotechnology is not a new science and it is not a new technology.“Nanotechnology is an enabling technology that allows us to develop materials with improved or totally new properties”It is rather an extension of the sciences and technologies already developed for many years ,to examine the nature of our world at an ever smaller scale.Nanotechnology is the use of very small particles of material. A nanometer is a billionth of a meter.The size of the particles, is very important because at the length scale of the nanometer, 10-9m, the properties of the material actually become affected.
2. Nanotechnology In Construction….
The construction business will inevitably be a beneficiary of this nanotechnology.In fact it already is in the fields of concrete, steel and glass,and many more. Concrete is stronger, more durable and more easily placed; steel is made tougher ; glass is self-cleaning.Paints are made more insulating and water repelling.
3. Introduction To Nano Materials Nano particle:
It is defined as a particle with at least one dimension less than 200nm.It is quantum dots if they are small enough (typically sub 10nm) such that jumps in energy levels occur.Nano composite : It is produced by adding Nano particle to a bulk material in order to improve the bulk material’s properties.
4. Carbon Nano Tubes (CNT)
They are cylindrical with nanometer diameter.They can be several millimeters in lengththey have 5 times the Young’s modulus and 8 times (theoretically 100 times) the strength of steel whilst being 1/6th the density.Thermal conduction is also very high along the tube axis
5. Titanium oxide
Titanium dioxide is a widely used white pigment.It can oxidize oxygen or organic materials, and so added to paints, cements, windows, tiles, or other products for sterilizing, deodorizing and anti-fouling propertiesWhen incorporated into outdoor building materials can substantially reduce concentrations of airborne pollutants. Additionally, as TiO2 is exposed to UV light, it becomes increasingly hydrophilic ,thus it can be used for anti-fogging coatings or selfcleaning windows.
6. NANOTECHNOLOGY IN CONCRETE
Concrete is a mixture of cement, sand(fine aggregate), coarse aggregate and water.As concrete is most usable material in construction industry it’s been require to improve its quality. The mechanical behavior of concrete materials depends on phenomena that occur on a micro and a nano scale.
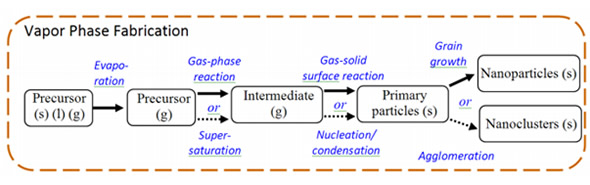
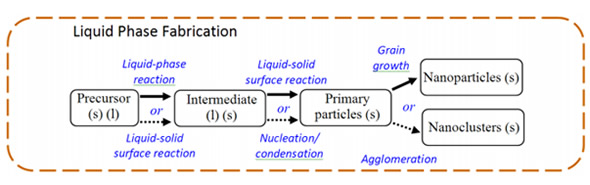
7. Nanotechnology in Concrete Nanotechnology can: modify the molecular structure of concrete material to improve the material's properties as shown in the chart.Nano-concrete is defined as “A concrete made with Portland cement particles that are less than 500 Nano-meters as the cementing agent”.
8. Concrete is, after all, a macro-material strongly influenced by its nano-properties.NANO-SILICA: particle packing in concrete can be improved by using nano-silica which leads to a densifying of the micro and nanostructure resulting in improved mechanical properties. Nano-silica addition to cement based materials can also control the degradation of the fundamental C-S-H (calcium-silicate hydrate) reaction of concrete caused by calcium leaching in water as well as block water penetration and therefore lead to improvements in durability. Related to improved particle packing, high energy milling of ordinary Portland cement (OPC) clinker and standard sand, produces a greater particle size diminution with respect to conventional OPC and, as a result, the compressive strength of the refined material is also 3 to 6 times higher.
9. If these Nano-cement particles can be processed with Nano tubes and reactive Nano-size silica particles, conductive, strong, tough and room temperature processed ceramics can be developed both for electronic applications and coatings. Average size of Portland cement particle is about 50 microns.In thinner final products and faster setting time, micro cement with a maximum particle size of about 5 microns is being used. Therefore is reduced to obtain nano-portland cement.Hydration tests indicated that the Nano-cement had a more rapid hydration rate than Portland cement.
10. TiO2 In Concrete TiO2 is a white pigment and can be used as an excellent reflective coating.it is hydrophilic and therefore gives self cleaning properties to surfaces to which it is applied. The process by which this occurs is that rain water is attracted to the surface and forms sheets which collect the pollutants and dirt particles previously broken down and washes them off. The resulting concrete, already used in projects around the world, has a white color that retains its whiteness very effectively unlike the stained buildings of the material’s pioneering past.
11. CNTs In ConcreteThe addition of small amounts (1% wt) of CNT’s can improve the mechanical properties of samples consisting of the main Portland cement phase and water.Oxidized multi-walled Nano tubes (MWNT’s) show the best improvements both in compressive strength (+ 25 N/mm2) and flexural strength (+ 8 N/mm2) compared to the samples without the reinforcement.A number of investigations have been carried out for developing smart concrete using carbon fibers.
12. NANOTECHNOLOGY AND STEEL
Need For Nanotechnology In Steel..Fatigue is a significant issue that can lead to the structural failure of steel subject to cyclic loading, such as in bridges or towers. This can happen at stresses significantly lower than the yield stress of the material and lead to a significant shortening of useful life of the structure.Stress risers are responsible for initiating cracks from which fatigue failure results and research has shown that the addition of copper Nanoparticle reduces the surface unevenness of steel which then limits the number of stress risers and hence fatigue cracking.Advancements in this technology would lead to increased safety, less need for monitoring and more efficient materials use in construction prone to fatigue issues.
13. Temperature restrictionAbove 750 F, regular steel starts to lose its structural integrity, and at 1100 F, steel loses 50 percent of its strength.A new formula infuses steel with nanoscale copper particles, this formula could maintain structural integrity at temperatures up to 1000 F.the new steel allows ultra-high strength to be combined with good formability, corrosion resistance and a good surface finish.
14. High Strength Steel Cables Current research into the refinement of the cementite phase of steel to a Nano-size has produced stronger cables. A stronger cable material would reduce the costs and period of construction, especially in suspension bridges .Sustainability is also enhanced by the use of higher cable strength as this leads to a more efficient use of materials.High rise structures require high strength joints and this in turn leads to the need for high strength bolts.
15. High strength bolts The capacity of high strength bolts is realized generally through quenching and tempering and the microstructures of such products consist of tempered martensite.When the tensile strength of tempered martensite steel exceeds 1,200 MPa even a very small amount of hydrogen embrittles the grain boundaries and the steel material may fail during use.vanadium and molybdenum Nanoparticle has shown that they improve the delayed fracture problems associated with high strength bolts, improving the steel micro-structure.
16. Two products in international marketSandvik NanoflexMMFX2 steelproduced by Sandvik Materials Technology(Sweden)desirable qualities of a high Young’s Modulus and high strength resistant to corrosion due to the presence of very hard nanometer-sized particlesThe use of stainless steel reinforcement in concrete structures is limited as it is cost prohibitive.produced by MMFX Steel Corp (America)has the mechanical properties of conventional steelhas a modified nano-structure that makes it corrosion resistantit is an alternative to conventional stainless steel, but at a lower cost.
17. NANOTECHNOLOGY AND GLASS (SELF CLEANING)
Vital role of glass in buildings The current state of the art in cladding is an active system which tracks sun, wind and rain in order to control the building environment and contribute to sustainability Consequently, there is a lot of research being carried out on the application of nanotechnology to glassMost of glass in construction is, on the exterior surface of buildings and the control of light and heat entering through glazing is a major issue. Research into nanotechnological solutions to this centers around four different strategies to block light& heat coming through windows.
18. Self cleaning glass using TiO2Titanium dioxide (TiO2) is usedin Nanoparticle form to coat glazing since it has sterilizing and anti-fouling properties. The particles catalyze powerful reactions which breakdown organic pollutants, volatile organic compounds and bacterial membranes. TiO2 is hydrophilic and this attraction to water forms sheets out of rain drops which then wash off the dirt particles broken down in the previous process. Glass incorporating this self cleaning technology is available on the market today.
19. Self cleaning glass. Fire and heat protection Fire-protective glass is another application of nanotechnology. This is achieved by using a clear intumescent layer sandwiched between glass panels (an interlayer) formed of fumed silica (SiO2) Nanoparticle which turns into a rigid and opaque fire shield when heated.For heat protection thin film coatings are being developed which are spectrally sensitive surface applications for window glass and filter out unwanted infrared frequencies of light (which heat up a room) and reduce the heat gain in buildings, however, these are effectively a passive solution. As an active solution, thermo chromic technologies are being studied which react to temperature and provide insulation to give protection from heating whilst maintaining adequate lighting.
20. Other technologies…A third strategy, that produces a similar outcome by a different process, involves photo chromic technologies which react to changes in light intensity.Electro chromic coatings are being developed that react to changes in applied voltage by using tungsten oxidelayer; thereby becoming more opaque atthe touch of a button. All these applications are intended to reduce energy use in cooling buildings.
21. NANO TECHNOLOGY IN OTHER DISCIPLINES
Nanotechnology and wood : Wood is also composed of nanotubes or “nanofibrils”, lignocelluloses are twice as strong as steel. Nanofibrils would lead to a new paradigm in sustainable construction Functionality onto lignocelluloses surfaces at the nanoscale could open new opportunities for such things as self-sterilizing surfaces, internal self-repair, and electronic lignocelluloses devices. Currently, however, research in these areas appears limited.Researchers have developed a highly water repellent coating based on the actions of the lotus leaf as a result of the incorporation of silica and alumina Nanoparticle and hydrophobic polymers.
Nanotechnology and coatings Nanotechnology is being applied to paints and insulating properties, produced by the addition of nano-sized cells, pores and particles, giving very limited paths for thermal conduction (R values are double those for insulating foam), are currently available.This type of paint is used, for corrosion protection under insulation since it is hydrophobic and repels water from the metal pipe and can also protect metal from salt water attack.
Elimination of toxic gases The absorption of carbon monoxide is done by using cuprous salt and adsorption of hydrocarbons is done by using a complex nanomaterial. i.e., Carbon Monolithic Aero gels.Production of Aero gels is done by sol-gel process.Adsorption capacity measurements show that modified hydrophobic Carbon aero gels are excellent adsorbents for different toxic organic compounds from water.
Conclusion In conclusion, nanotechnology offers the possibility of great advances whereas conventional approaches, at best, offer only incremental improvements.“At this moment the main limitation is the high costs of nanotechnology. Also concerns with the environmental effects”The waves of change being propagated by progress at the nanoscale will therefore be felt far and wide and nowhere more so than in construction due its large economic and social presence.